Finland ends use of AstraZeneca vaccine in November
Published : 19 Oct 2021, 01:43
Updated : 19 Oct 2021, 01:48
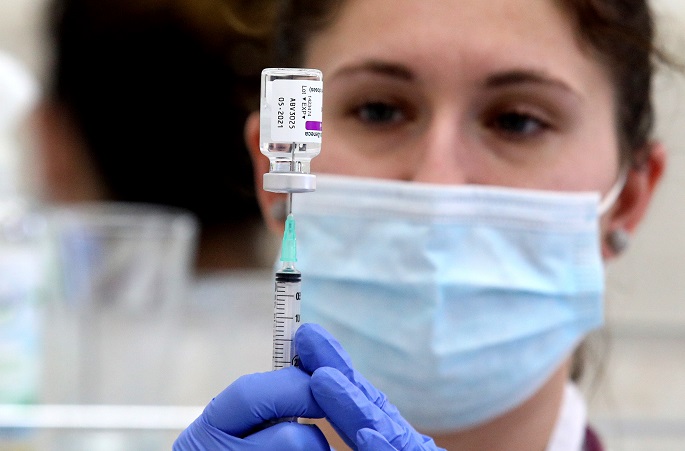
The use of the AstraZeneca Vaxzevria coronavirus vaccines will end in Finland when the current Vaxzevria vaccines expire on 30 November, said the National Institute for Health and Welfare (THL) in a press release on Monday.
After this the vaccine will not be procured for Finland. Before their expiration, the vaccine will still be available as before for second and third vaccine dose.
At present, the AstraZeneca vaccines are being offered only to those over the age of 65. Vaxzevria is an adenovirus vector vaccine.
“Starting next week, the AstraZeneca Vaxzevria vaccines will no longer be offered as the first vaccine because this same vaccine will not be available as a required second dose six weeks later because the lot expires”, said Mia Kontio, Leading Expert at THL.
Starting in the last week of October, the Janssen adenovirus vector vaccine can be offered in place of the AstraZeneca vaccine to those aged 65 and over, in accordance with a national recommendation.
The vaccine can also be offered to those between the ages of 18 and 64 who cannot be given an mRNA vaccine for medical reasons.
“An mRNA vaccine is suitable for nearly everyone. In rare cases when someone who has received an mRNA vaccine has suffered an anaphylactic reaction from a vaccine or one of its components, an mRNA vaccine cannot be given as the second dose. In such a situation, an adenovirus vaccine must be used”, Kontio said.
The same safety considerations that apply to vaccine manufactured by AstraZeneca also apply to the Janssen vaccine. In accordance with the precautionary principle, the Janssen vaccine is also not being offered to those under the age of 65 because of a very rare risk of thrombosis.
“The most prevalent vaccine among those coming to Finland is the Cominarty vaccine from Biontech-Pfizer, which is an mRNA vaccine. It is the vaccine that will be offered to most of the population in the future. If someone over the age of 65 does not want to take an mRNA vaccine, the Janssen adenovirus vector vaccine can be offered”, Kontio added.
The Janssen vaccine is very similar to the AstraZeneca vaccine in its effectiveness. It is currently licensed for use in a basic series of a single vaccine dose, but research has already been conducted on a series of two doses.
The manufacturer is currently applying to pharmaceutical authorities for a sales licence for use as a booster, as the protective effect of a single dose deteriorates as is the case with the other coronavirus vaccines.
For now, THL is also recommending a booster vaccination six months after the first dose for a vaccine series that started with Janssen.
THL has instructed municipalities and hospital districts to stop using the AstraZeneca vaccine.